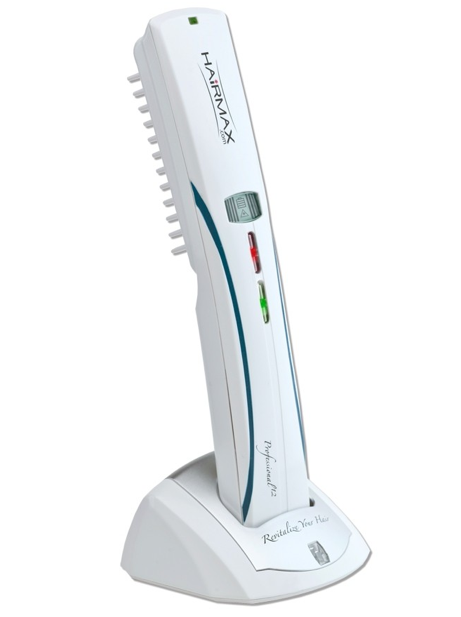
Ofrecen en el mercado muchos dispositivos laser con el propósito de estimular el crecimiento capilar. Hairmax laser comb es el único láser que tiene estudios científicos que demuestran efectividad en los pacientes que lo han utilizado. Es el primer y único dispositivo de luz láser aprobado por la FDA en estados unidos que promueve el crecimiento del pelo tanto en hombres como en mujeres.
El láser funciona produciendo una luz con una longitud de onda específica que llega directamente al folículo piloso. Esta luz es captada por las células encargadas de producir el pelo y la transforman en energía para su beneficio. Esto se traduce en que el pelo mejora su grosor y calidad. Son necesarias 3 sesiones semanales de 15 minutos de duración. Los cambios comienzan a notarse entre la 6 – 12 semana.
El láser emite una luz de baja intensidad que proporciona una fuente de energía adicional al folículo piloso. La fototerapia láser estimula la producción celular de factores de crecimiento que harán entrar y mantener a la mayoría de folículos pilosos en fase anágena o de crecimiento.
El fabricante recomienda sesiones de 10 – 15 minutos, se deben hacer 3 veces por semana en días no consecutivos y los resultados se podrán ver a partir del 4to mes. Es indispensable continuar con el tratamiento pues si se para se perderá el volumen recuperado. El tratamiento es seguro y eficaz y no tiene contraindicaciones.
Nuestra RecomendaciónEntusiasmados por la certificación FDA, por la teoría de cómo es su funcionamiento y ante los resultados vistos en algunos pacientes decidimos investigar un poco más antes de recomendarlo o de censurarlo, en especial por la presencia también de un número significativo de personas inconformes.
A continuación está el texto original de aprobación por la FDA para su uso en el tratamiento de alopecia androgénica en hombres y mujeres. La conclusión de HairDoctors, es que los resultados son reales pero algo pobres y requieren tratamientos prolongados, así que si usted tiene la paciencia, los recursos y el tiempo para este tipo de tratamiento, vale la pena intentarlo; sino, use tratamientos con mayor eficacia como los medicamentos descritos arriba.
FDA Clearance for HairMax Laser Comb
Date of Submission: June 16, 2011
Proprietary Name: HairMax Dual 12
Common Name: Lamp, non-heating, for promotion of hair growth
Regulatory Class: L1
Product Codes: OAP
Predicate Device(s): Lexington International, LLC – HairMax Lux 9 and HairMax Pro 12 (K103368) and Female Lux 9 (KI 110233)
[toggle title=”Leer Más▼”]
Device Description:
HairMax is a hand-held low-level laser device that emits laser light with the intention to promote hair growth. -The device provides distributed laser light to the scalp while the comb teeth simultaneously part the user’s hair to ensure maximum laser light reaches the user’s scalp. HairMax Dual 12 utilizes twelve laser modules, six with wavelengths of 655nm and six with wavelengths of 635n with the same exact output power as the HairMax Pro 12.
Intended Use:
The HairMax Dual 12 is indicated to Treat Androgenetic Alopecia and Promote Hair Growth in Females who have Ludwig (Savin) Scale 1-4, 11-1, H1-2, or frontal and Fitzpatrick Skin Types I to IV.
Technological Characteristics:
The HairMax Dual 12 consists of a band-held low-level laser device that promotes hair growth. The device provides distributed laser light to the scalp while the device’s comb teeth simultaneously part the user’s hair to ensure maximum laser light reaches the user’s scalp. When in use, the device emits a beep and vibration every four seconds to notify the user to move the device to a new section of the scalp.
Performance Testing:
Testing to IfiC 60601-1 and 60601-1-2 confirm the device’s adherence to LVD,
electrical and EMC safety requirements.
Clinical Testing:
A randomized, double-blind, controlled, multi-center clinical trial was conducted at 3 sites, Cleveland Clinic – Wilma Bergfeld M.D., University of Minnesota – Maria
Hordinsky M.D. and University of Miami – Lawrence Schachner M.D. Each site complied with Institutional Review Board approval and oversight and in accordance with applicable references defined by the Food and Drug Cosmetics Act and Title 21, Code of Federal Regulations.
The clinical trials were listed on www.clinicaltrials.gov.
The purpose of the clinical trial was to confirm the performance of the HairMax Dual 12 to treat androgenetic alopecia and promote hair growth in females who have
Ludwig (Savin) Scale 1-4, 11- 1, H-2, or frontal and Fitzpatrick Skin Types I to IV.
After 16 weeks of treatment, 81% of the subjects using the HairMax Dual 12 experienced increases in hair count, (based on a minimum of 5 new hairs being observed at follow up). Benefits continued to improve after 26 weeks of treatment, 95% of the subjects using the HairMax Dual 12 experienced significant increases in hair count (based on a minimum of 5 new hairs being observed at follow up).
No subjects experienced any serious adverse event from the treatments.
The study population included females between the ages of 25 to 60 years with a diagnosis of androgenetic alopecia who had been experiencing active hair loss within the last 12 months. They were also required to have a Ludwig (Savin) classification of 1-4 or Frontal, and have Skin Type I, II, III, or IV on the Fitzpatrick Skin Type Scale. Skin types were limited to the Fitzpatrick Skin types I-IV to facilitate the hair counting process, as it is difficult to count hairs on darker skin tones,
Substantial Equivalence
The HairMax Dual 12 is identical in technological characteristics as the HairMax Pro 12 as cleared in K103368 and HairMax Lux 9 for females (KL 10233), except for the dual wavelength component. The HairMax Pro 12 contains twelve 655nm laser modules, the HairMax Dual 12 contains six 655nm laser modules and six 635nm laser modules.
All other technical aspects, including its power output, its comb component, its instructions for use and its audible or vibrating timer remain identical. The modification to the HairMax Dual 12 does not change the intended use of the product nor does it affect the products fundamental scientific technology. Therefore this change does not raise new questions of safety or effectiveness.
This was also demonstrated in a randomized, double-blind, control clinical study evaluating changes in terminal hair-count in the evaluation zone, as well as usability studies to validate instructions for use, confirm that device modifications do not affect the safe and effective use of the devices when compared to the predicates.
For those reasons, HairMax Dual 12 satisfies FDA’s substantial equivalence with respect to both the intended use and technological characteristics.
[/toggle]